The pharmaceutical giant has admitted its vaccine can cause ‘vaccine-induced immune thrombotic thrombocytopenia’—dangerous blood clots—in some patients.
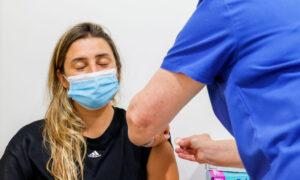
Pharmaceutical manufacturer AstraZeneca has accepted for the first time that its COVID-19 vaccine can, “in very rare cases,” cause thrombosis with thrombocytopenia syndrome (TTS), which is characterised by blood clots (thrombosis) and low red blood cell counts (thrombocytopenia). It is also called vaccine-induced immune thrombotic thrombocytopenia (VITT).
This admission was made in a legal document submitted to the High Court in the UK, where the company is defending against a class action lawsuit alleging that its COVID-19 vaccine led to several deaths and serious injuries.
Last year, Jamie Scott, a father of two, filed a complaint against the British-Swedish multinational. He claimed that after receiving the vaccine in April 2021, he developed a blood clot resulting in severe brain impairment. At one point, the hospital informed his wife that he might not survive.
Mr. Scott alleges that the pharmaceutical giant overstated the vaccine’s effectiveness and downplayed its risks.
Blood Clot in the Brain
Ten days after his first dose, Mr. Scott woke up with a severe headache, started vomiting, and had trouble speaking. He was taken to hospital where he was diagnosed with a clot that was stopping blood from draining from his brain, as well as a haemorrhage in the brain itself. He had surgery and was in a coma for just over a month.
Mr. Scott has been left with poor memory, trouble reading, writing, listening, and speaking, is partially blind in both eyes, and suffers from pain and fatigue.
AstraZeneca now faces a class action with 51 patients and families alleging a link between the vaccine, known as Vaxzevria, and the rare condition and claiming damages of over £100 million (US$125,000), though Mr. Scott’s claim is expected to be heard first.
The claimants are pursuing a two-pronged strategy: taking legal action under the UK Consumer Protection Act 1987 as well as claiming payment under the government-run Vaccine Damage Payment Scheme.
The scheme has paid out in several cases but is limited to £120,000 per claim, and applicants must prove severe disablement.
AstraZeneca Denied Causal Link
Responding to Mr. Scott’s lawyers in May 2023, the company said, “We do not accept that TTS is caused by the vaccine at a generic level” but added, “Our sympathy goes out to anyone who has lost loved ones or reported health problems.”
“From the body of evidence in clinical trials and real-world data, Vaxzevria has continuously been shown to have an acceptable safety profile and regulators around the world consistently state that the benefits of vaccination outweigh the risks of extremely rare potential side effects,” the company said.
Even though the legal claim is against AstraZeneca, the UK taxpayer will have to pay any compensation awarded under an indemnity agreement that the government gave the company early in the pandemic.
One of two lawyers handling the claims, Peter Todd, a consultant solicitor with Scott-Moncrieff & Associates, said the complications associated with the vaccine included stroke, heart failure, and leg amputations. He said the technology involved in the AstraZeneca vaccine was “risky.”
According to Sarah Moore, a partner in the second legal firm, Hausfeld, damages awarded in the case could run into millions of pounds per claimant.
“We’ve been trying to get the government to reform their statutory scheme,” she said.
“We didn’t want to litigate but the government has forced us into a corner. The only way these families can get compensation is to fight the battle they didn’t want to fight.”
AstraZeneca Removes Vaccine from Australian Register
AstraZeneca manufactured the Oxford University vaccine on a not-for-profit basis and claimed it would deliver up to 3 billion doses of its vaccine across the globe by the end of 2021, just 18 months after development had begun.
An independent study by disease-forecasting company Airfinity, published in 2022, estimated the vaccine had saved more than six million lives in its first year of use, more than any other COVID-19 preventative.
In Australia, AstraZeneca Pty Ltd voluntarily removed Vaxzevria (previously known as COVID-19 Vaccine AstraZeneca) from the Australian Register of Therapeutic Goods in late April 2024.
According to the Therapeutic Goods Administration (TGA), Australia’s regulatory authority for therapeutic goods, this move was “a business decision of the company, due to no current or anticipated future demand of the vaccine, and follows similar business decisions made overseas.”
Reliant on Pharma Funding
In most countries, medicines regulators such as the TGA are primarily funded by the pharmaceutical industry, intended to support the cost of swiftly reviewing drug applications.
According to a recent comparison by the British Medical Journal, Australia’s regulator relies heavily on these fees, covering 96 percent of its operating costs, compared to 65 percent for the US Food and Drug Administration and 50.5 percent for Health Canada. The European Medicines Agency is closest to Australia’s figure, at 89 percent.
The closest to Australia’s figure is the European Medicines Agency, at 89 percent.
Members of the TGA approvals panel also had the second-highest declared conflicts of interest with the manufacturers of COVID-19 vaccines. Fifty percent declared such a conflict; 75 percent of Japan’s regulators had an issue. That compares to less than 10 percent in the U.S., three percent in Europe, and none in Canada.
Australia and Canada were the only jurisdictions in the sample that did not routinely publish members’ conflicts of interest as public information.
In 2020/21, the TGA approved more than nine of every 10 drug company applications, the second highest rate behind Europe.













 English (US) ·
English (US) ·  Turkish (TR) ·
Turkish (TR) ·